Researchers in the United States published a study in the journal PLOS Pathogens that examined the shedding of infectious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virions despite vaccination against it.
 Study: Shedding of infectious SARS-CoV-2 despite vaccination. Image Credit: MrSquid / Shutterstock
Study: Shedding of infectious SARS-CoV-2 despite vaccination. Image Credit: MrSquid / Shutterstock
Background
The SARS-CoV-2 Delta variant was first identified in March 2021 and was linked to an upsurge in the incidence of coronavirus disease 2019 (COVID-19) infection in North America in the summer of 2021. The surge of cases linked to viruses of the Delta lineage was the first notable rise in SARS-CoV-2 infection rates after COVID-19 vaccines became readily accessible in the US.
By July 2021, SARS-CoV-2 infection rates were low in the United States, and national and local public health organizations were relaxing regulations on the use of face masks and other non-pharmaceutical measures to stop the spread of the virus. Whether individuals infected with SARS-CoV-2, notwithstanding vaccination, could spread the infection to others was a crucial consideration in developing these policies.
About the study
In the present study, researchers evaluated the SARS-CoV-2 ribonucleic acid (RNA) burden in nasal swabs obtained from vaccinated and unvaccinated people to ascertain if people with vaccine breakthrough infections might shed SARS-CoV-2 Delta viruses at levels compatible with the potential transmission.
The anterior nasal swab samples sent for clinical testing to a commercial reverse transcription-polymerase chain reaction (RT-PCR) testing service between 28 June 2021 and 1 December 2021 were employed. The study utilized sample-associated metadata to estimate viral RNA burden in people who tested positive for SARS-CoV-2 during a period of high Delta variant prevalence and its association with the person’s vaccination status. At numerous clinic locations throughout Wisconsin, samples were obtained using standardized collection kits from patients who required SARS-CoV-2 RT-PCR testing. To assess the nasal viral RNA load, the team analyzed RT-PCR cycle threshold (Ct) data related to 20,431 specimens from completely vaccinated or unvaccinated people.
The levels of Ct were determined using the Flu-SC2 Multiplex Assay. This RT-PCR method can simultaneously identify influenza A and B and SARS-CoV-2 nucleic acid in anterior nasal swabs. Reverse transcription into complementary deoxyribonucleic acid (cDNA) and amplification was performed on the RNA obtained from anterior nasal swab samples. A no-template control, a positive extraction control containing human RNAse P, and an internal RNAse P control were all used as controls.
If vaccine registry or self-reported data showed that a final vaccine dosage was received at least 14 days before the submission of a SARS-CoV-2 positive, the individual was deemed completely vaccinated at the time of testing. The team evaluated the presence of an infectious virus and observed the presence of cytopathic effects throughout the course of five days using an initial batch of specimens with Ct values less than 25. Samples were selected by employing N1 Ct-matching between unvaccinated and completely vaccinated individuals.
Results
Infected SARS-CoV-2 have previously been linked to SARS-CoV-2 RT-PCR Ct values below 25. Ct values less than 25 were observed in 6,253 of 9,347 fully vaccinated individuals and 6,739 of 11,084 unvaccinated people. The researchers derived standardized differences, which are the average differences between the groups divided by the pooled standard deviations, to determine the size of the differences between groups.
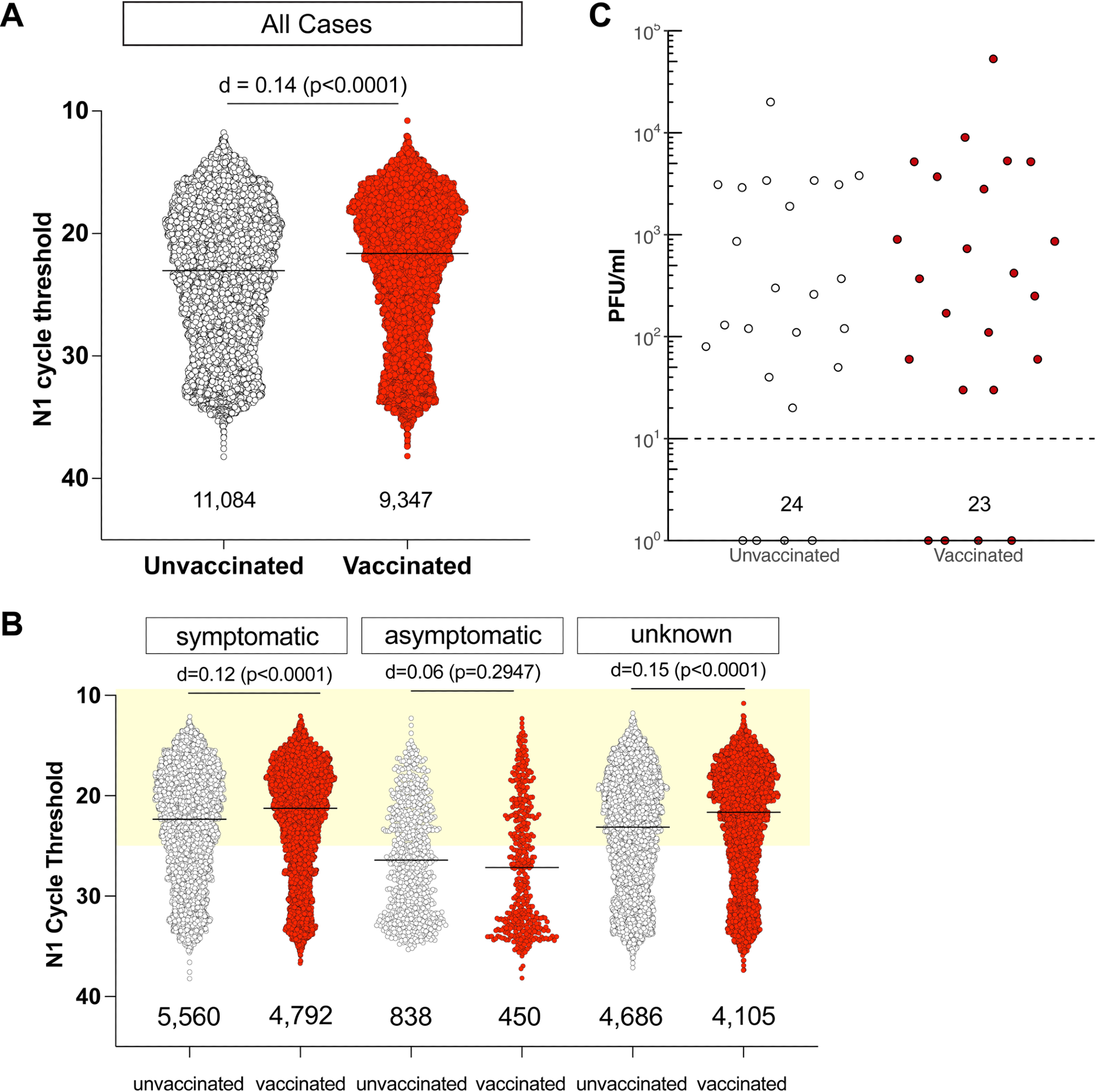 Individuals infected with SARS-CoV-2 despite full vaccination have low Ct values and shed similar amounts of infectious virus as unvaccinated individuals. A. N1 Ct values for SARS-CoV-2-positive specimens were grouped by vaccination status. RT-PCR was performed by Exact Sciences Corporation, responsible for over 10% of all PCR tests in Wisconsin during this period, using a qualitative diagnostic assay targeting the SARS-CoV-2 N gene (oligonucleotides identical to CDC’s N1 primer and probe set) that has been authorized for emergency use by FDA (https://www.fda.gov/media/138328/download)). An effect size of d< 0.2 is negligible. The number of samples in each group is listed under the dot plot. B. N1 Ct values for SARS-CoV-2-positive specimens grouped by vaccination status for individuals who were symptomatic or either asymptomatic or did not have any information, at the time of testing. Light yellow box indicates Ct values <25. C. We performed plaque assays on Vero E6 TMPRSS2 cells on a subset of specimens. Specimens were selected by N1 Ct-matching between fully vaccinated and unvaccinated persons. Specimens from individuals with unknown vaccination status were excluded from the analysis. Infectious titers are expressed as plaque-forming units (PFU) per milliliter of specimen. Specimens underwent a freeze-thaw cycle prior to virus titration.
Individuals infected with SARS-CoV-2 despite full vaccination have low Ct values and shed similar amounts of infectious virus as unvaccinated individuals. A. N1 Ct values for SARS-CoV-2-positive specimens were grouped by vaccination status. RT-PCR was performed by Exact Sciences Corporation, responsible for over 10% of all PCR tests in Wisconsin during this period, using a qualitative diagnostic assay targeting the SARS-CoV-2 N gene (oligonucleotides identical to CDC’s N1 primer and probe set) that has been authorized for emergency use by FDA (https://www.fda.gov/media/138328/download)). An effect size of d< 0.2 is negligible. The number of samples in each group is listed under the dot plot. B. N1 Ct values for SARS-CoV-2-positive specimens grouped by vaccination status for individuals who were symptomatic or either asymptomatic or did not have any information, at the time of testing. Light yellow box indicates Ct values <25. C. We performed plaque assays on Vero E6 TMPRSS2 cells on a subset of specimens. Specimens were selected by N1 Ct-matching between fully vaccinated and unvaccinated persons. Specimens from individuals with unknown vaccination status were excluded from the analysis. Infectious titers are expressed as plaque-forming units (PFU) per milliliter of specimen. Specimens underwent a freeze-thaw cycle prior to virus titration.
We found no discernible correlation between Ct values in infected individuals and vaccination status. Irrespective of whether they had symptoms at the time of testing, vaccinated persons had low Ct values, with Ct values less than 25 being found in 65% of symptomatic unvaccinated individuals and in 70% of completely vaccinated symptomatic patients.
Notably, the interval between the start of symptoms and testing was unaffected by vaccination status for patients with symptoms. In our cohort, both vaccinated and unvaccinated people reported a median delay of 2.4 days between the beginning of symptoms and testing. In this study sample, 92% of people sought testing within six days of the onset of symptoms.
Conclusion
The study findings showed that a significant fraction of those who developed SARS-Cov-2 Delta virus infections after receiving vaccinations had low Ct values compatible with the possibility of shedding infectious viruses. The results indicated that people who are infected despite receiving vaccinations could spread SARS-CoV-2. In order to stop transmission, infection prevention is essential. The researchers believe that those who have and have not received the COVID-19 vaccine should continue to follow non-pharmaceutical measures to limit the transmission of COVID-19.