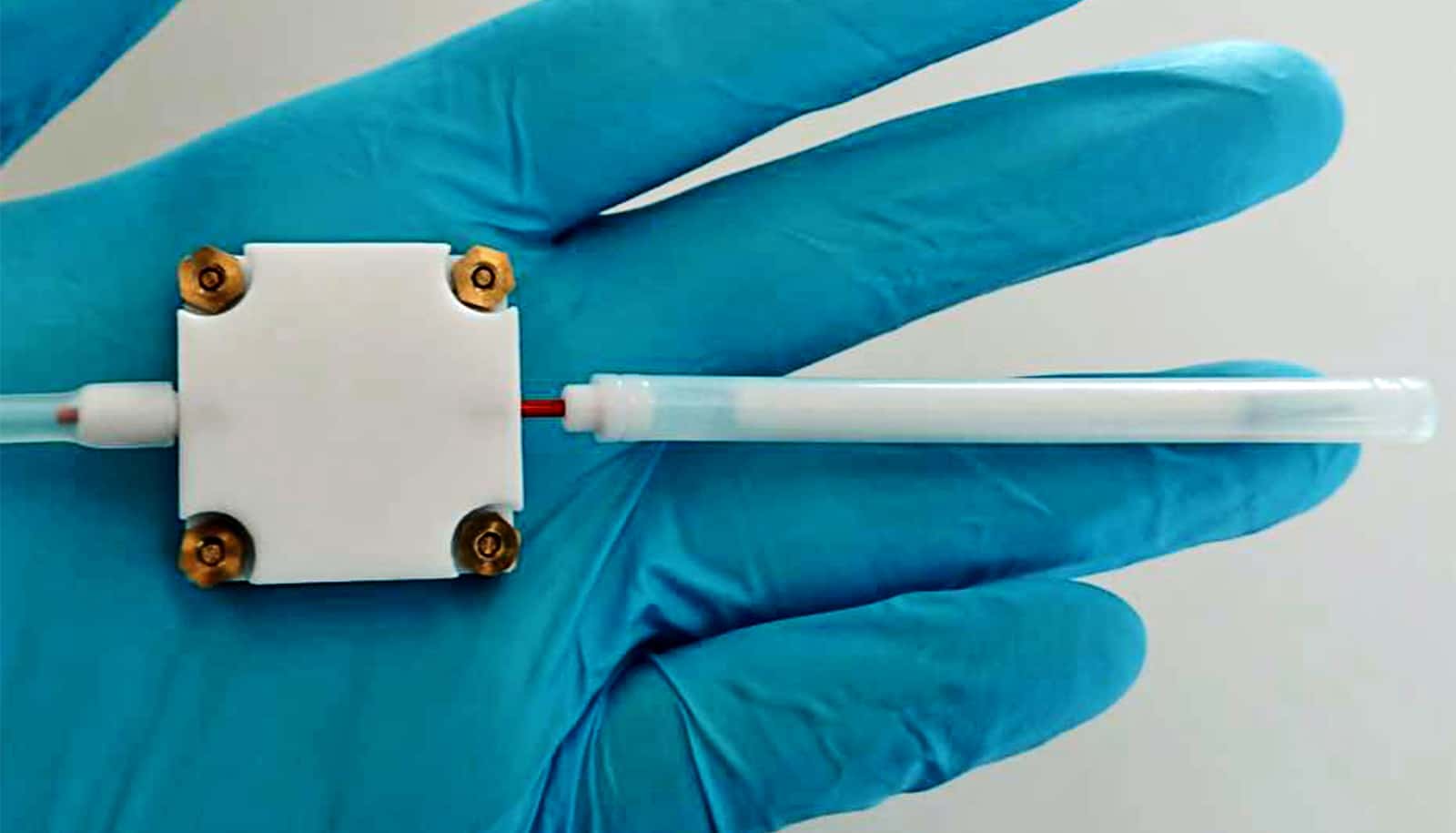
A new, affordable handheld device can detect tainted alcohol in two minutes by distinguishing between methanol and ethanol vapors in a beverage, researchers report.
Researchers say the device can also analyze a patient’s breath to diagnose methanol poisoning, ensuring that health workers take appropriate measures in an emergency without delay.
Some refer to methanol as ethanol’s deadly twin. The latter is the intoxicating ingredient in wine, beer, and schnapps, but the former is a chemical that becomes highly toxic when the human body metabolizes it.
Even a relatively small amount of methanol can cause blindness or even prove fatal if left untreated.
Cases of poisoning from the consumption of alcoholic beverages tainted with methanol occur time and again, particularly in developing and emerging countries, because alcoholic fermentation also produces small quantities of methanol.
Whenever alcohol is unprofessionally distilled in backyard operations, relevant amounts of methanol may end up in the liquor. Beverages adulterated with windscreen washer fluid or other liquids containing methanol are another potential cause of poisoning.
Beyond breathalyzers
Until now, the only way to distinguish methanol from ethanol was in a chemical analysis laboratory. Even hospitals require relatively large, expensive equipment in order to diagnose methanol poisoning.
“These appliances are rarely available in emerging and developing countries, where outbreaks of methanol poisoning are most prevalent,” says Andreas Güntner, a research group leader at the Particle Technology Laboratory of ETH Zurich Professor Sotiris Pratsinis and a researcher at the University Hospital Zurich.
The new device, which is based on a small metal oxide sensor, offers a potential solution.
There’s nothing new about using metal oxide sensors to measure alcoholic vapors. However, this method couldn’t distinguish between different alcohols, such as ethanol and methanol.
“Even the breathalyzer tests used by the police measure only ethanol, although some devices also erroneously identify methanol as ethanol,” says Jan van den Broek, a doctoral student and lead author of the study in Nature Communications.
First, the researchers developed a highly sensitive alcohol sensor using nanoparticles of tin oxide doped with palladium. Next, they used a trick to differentiate between methanol and ethanol. Instead of analyzing the sample directly with the sensor, researchers first separated the two types of alcohol in an attached tube filled with a porous polymer, through which a small pump sucked the sample air. As its molecules are smaller, methanol passes through the polymer tube more quickly than ethanol.
The measuring device proved exceptionally sensitive. In laboratory tests, it detected even trace amounts of methanol contamination selectively in alcoholic beverages, down to the low legal limits. Furthermore, the scientists analyzed breath samples from a person who had previously drunk rum. For test purposes, the researchers subsequently added a small quantity of methanol to the breath sample.
Tank leaks, too
The researchers have filed a patent application for the measuring method and are now working to integrate the technology into a device that they can put to practical use.
“This technology is low cost, making it suitable for use in developing countries as well. Moreover, it’s simple to use and can be operated even without laboratory training, for example by authorities or tourists,” Güntner says. It is also ideal for quality control in distilleries.
Methanol is more than just a nuisance in conjunction with alcoholic beverages, it is also an important industrial chemical—and one that might come to play an even more important role: scientists are considering it as a potential future fuel, since methanol fuel cells can power vehicles. The technology could also be useful as an alarm sensor to detect leaks in tanks.
Source: ETH Zurich